One year after the initiation of Phase I, Debiopharm announced the start of clinical phase II for Debio 1450 in ABSSSI and presentation of new data at ECCMID
Lausanne, Switzerland – April 23, 2015 – Debiopharm International SA (Debiopharm), part of Debiopharm Group™, a Swiss-based global biopharmaceutical company, today announced the start of a clinical phase II study to evaluate Debio 1450 for the treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSI) due to Staphylococci, including all resistant Staphylococcus strains, tested so far.
This study is designed as a dose-response study to evaluate the efficacy of Debio 1450 in both intravenous (IV) and oral formulations versus intravenous vancomycin switched to oral linezolid in the treatment of ABSSSI. Safety and tolerability of both the IV and oral formulations of Debio 1450 will be evaluated by conventional endpoints.
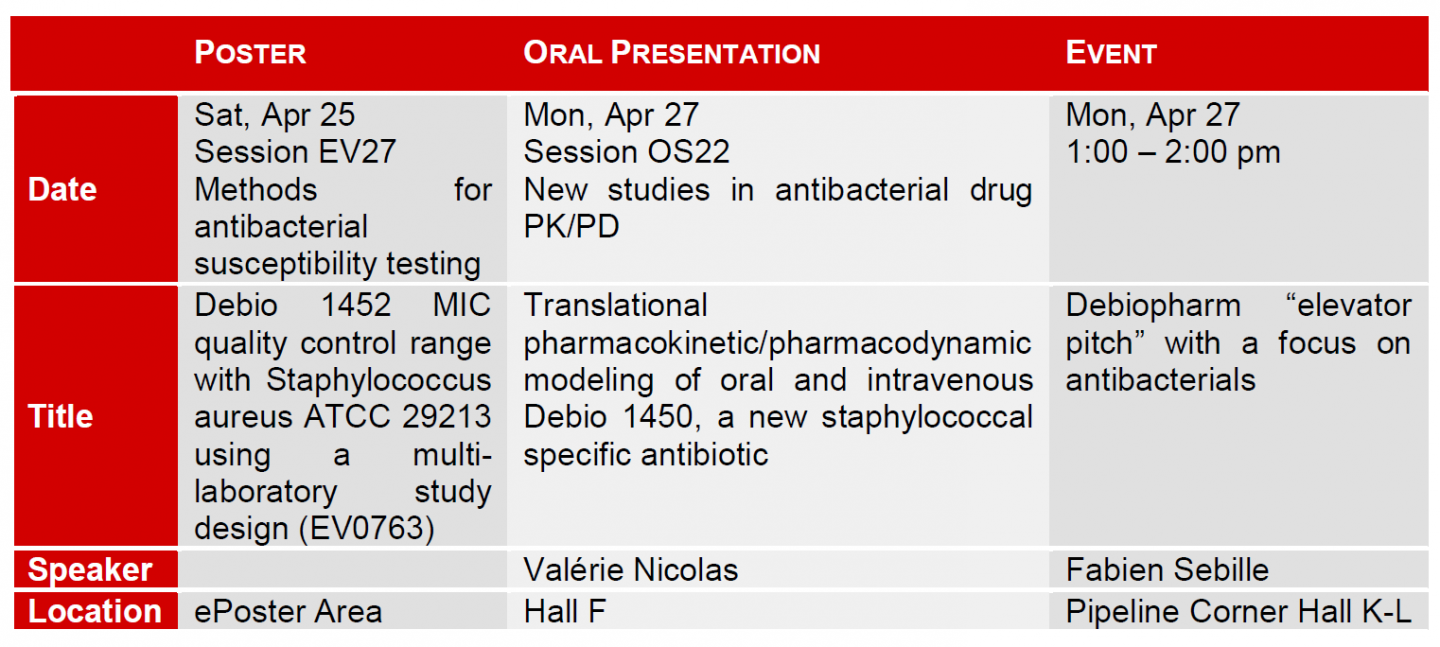
Moreover, Debiopharm will present data regarding Debio 1450 PK/PD at the 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), which will be held from 25 – 28 April 2015 in Copenhagen.
During ECCMID, Debiopharm will also take part in the Pipeline Corner project, a unique event organized on behalf of the European Society of Clinical Microbiology and Infectious Diseases, allowing companies to present their innovative anti-infective projects and meet stakeholders in this field. Please visit us at Pipeline Corner: Hall K – L
“Only one year after the enrollment of the administration of the first patient in Phase I, we are happy to announce the initiation of clinical PhII in ABSSSI”, said Dr Jean-Maurice Dumont, Vice President, Medical Affairs, Debiopharm International. “It emphasizes our commitment to develop highly valuable targeted antibiotics in order to alleviate problems of acquired resistance linked to broad-spectrum antibiotic usage”.
About Debio 1450
Debio 1450 is a prodrug of Debio 1452, it is a highly potent anti-infective agent that is specifically active against all Staphylococcus species, including all known resistant strains such as methicillin-resistant S. aureus (MRSA) and vancomycin-intermediate S. aureus (VISA). Oral and IV Debio 1450 formulations are being developed for clinical use in several serious infections. Moreover Debio 1452 had a successful phase IIa with 103 patients in ABSSSI.
About Debiopharm International SA
Debiopharm Group™ is a Swiss-based global biopharmaceutical group of four companies active in drug development, GMP manufacturing of proprietary drugs, diagnostics, and investments. Debiopharm International SA is focused on the development of prescription drugs that target unmet medical needs. The company in-licenses, develops and/or co-develops promising biological and small molecule drug candidates for global registration. The products are commercialized through out-licensing to pharmaceutical partners to give access to the largest number of patients worldwide.
For more information, please visit: www.debiopharm.com
We are on Twitter. Follow us @DebiopharmNews at http://twitter.com/DebiopharmNew
