Debio 0228/0328
ITM-91/ ITM-94D (Debio 0228/0328) is an investigational theranostic pair originally discovered by 3B Pharmaceuticals GmbH and now exclusively licensed to ITM. ITM-94D (Debio 0328) ([68Ga]Ga-DPI-4452) is a PET imaging agent that may be used independently and is designed to identify patients whose cancers overexpress CA IX. Once identified, these patients may be treated with the lutetium-labelled radioligand, ITM-91 (Debio 0228) ([177Lu]Lu-DPI-4452), which is designed to deliver targeted radiation to the tumor with the aim to destroy it from the inside. The recently launched GaLuCi™ study, a multi-center, non-randomized phase 1/2 clinical trial, is investigating the theranostic pair in patients with locally advanced renal, pancreatic, and colorectal cancer.
Product Snapshot
Radioligand Therapy Targeting CA IX
Delivery of high-dose radiation to tumors
Being researched in:
- Clear Cell Renal Cell Carcinoma
- Pancreatic Duodenal Adenocarcinoma
- Colorectal Cancer
In short
Initially discovered by the German biotech, 3B Pharmaceuticals (3BP), the peptide-based ligand demonstrates high selectivity and specificity for CA IX.
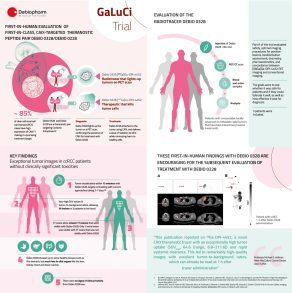
The first in human phase 1/2 GaLuCi™ study investigates the safety, tolerability and performance of the imaging compound, Debio 0328 and the therapeutic agent, Debio 0228.
Focus on the Mode of Action
The Debio 0228/0328 theranostic pair avoids damaging healthy tissues by delivering radioactive particles directly inside the tumor via the CA IX surface protein. Traveling through the bloodstream, the diagnostic radioligand lights up the tumor if the receptor is present, confirming the therapeutic radioligand can be used to treat the patient. The therapeutic radioligand attaches to the CA IX surface protein and delivers the radiation, minimizing the effects on healthy organs, and offering personalized, targeted cancer therapy.
Focus on the Theranostic Approach
This specific targeted approach, known as a “theranostic” approach, refers to the diagnosis and treatment of cancers using radiotracers. Through this two-pronged approach, potential responders are qualified for their personalized therapy via diagnostic molecular imaging evaluating target expression in their tumor lesions, allowing only those patients, who might potentially benefit, to receive the treatment.

Focus on the GaLuCi™ phase 1/2 trial
Patients with ccRCC, PDAC, and CRC
The GaLuCi™ trial is the first-in-human, multicenter, non-randomized phase 1/2 clinical trial assessing safety and tolerability, imaging characteristics, and the efficacy of the theranostic pair Debio 0228/0328 in patients with unresectable, locally advanced, or metastatic solid tumors. This theranostic trial is being carried out in three stages. The ongoing Part A is evaluating the safety and performance of the imaging agent in detecting CA IX-expressing solid tumors. Part B will assess escalating doses of the therapeutic agent, Debio 0228 in patients, whose tumors show high uptake of imaging tracer. Finally, based on the recommended dose from part B, Part C of the study will further assess the safety and preliminary efficacy of Debio 0228 in clear cell renal cell carcinoma (ccRCC), pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC).
For a closer look, copy and paste this link in your search browser: https://www.debiopharm.com/wp-content/uploads/2020/03/Debio-0228_Publication-infographic_20240528_v3.0-FINAL.pdf
Development milestones
-
2020
In-licenced by Debiopharm
-
2023
First-in human GaLuCi Trial
-
2024
Worldwide license granted to ITM
