Michael S. Hofman1,2, Ben Tran1,2, Darren R. Feldman3, Anna Pokorska-Bocci4, Solen Pichereau4, Jonathan Wessen4, Mohammad B. Haskali1,2, Richard B. Sparks5, Olena Vlasyuk4 and Ivana Galetic3
1 Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia
2 Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Victoria, Australia
3 Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, New York, New York
4 Debiopharm International SA, Lausanne, Switzerland
5 CDE Dosimetry Services, Inc., Knoxville, Tennessee
Journal of Nuclear Medicine February 2024, jnumed.123.267175; DOI
Abstract
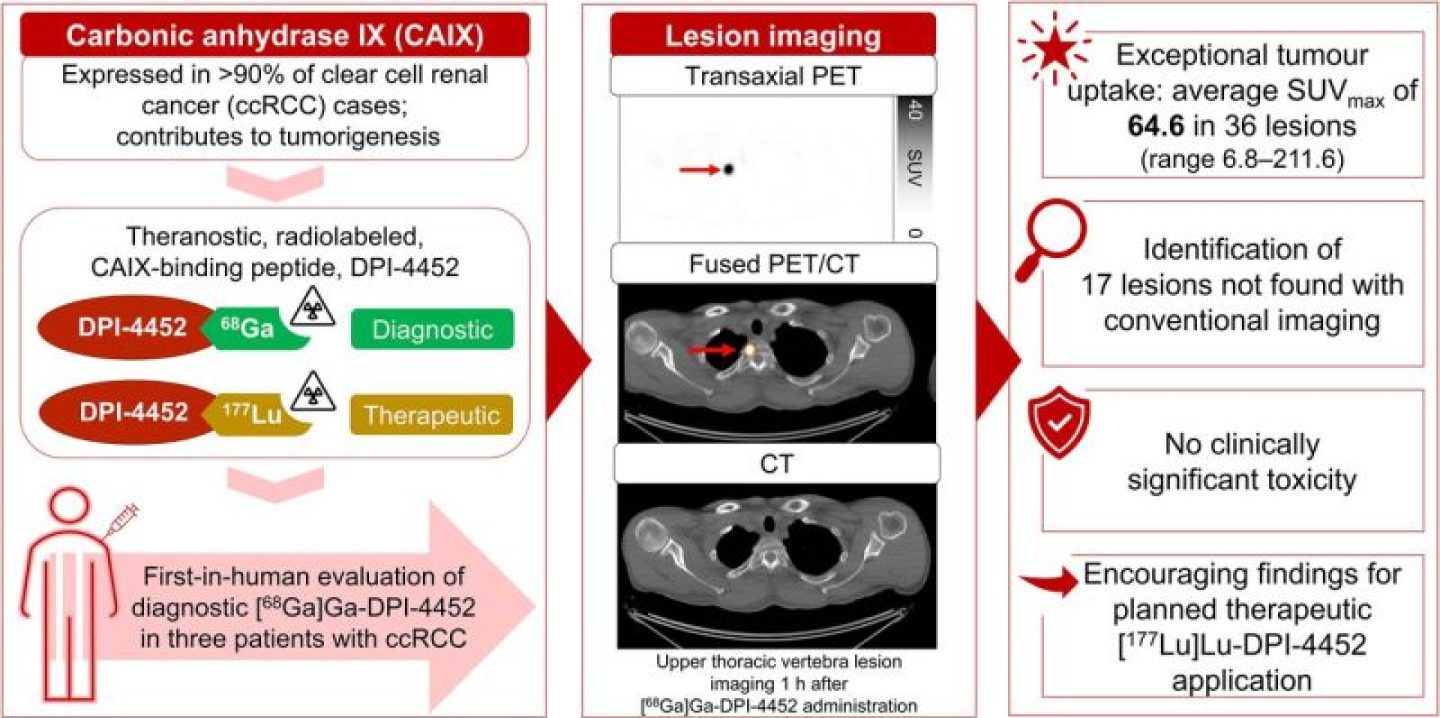
[68Ga]Ga-DPI-4452, a first-in-class carbonic anhydrase IX–binding radiolabeled peptide, is the imaging agent of a theranostic pair with [177Lu]Lu-DPI-4452, developed for selecting and treating patients with carbonic anhydrase IX–expressing tumors. Here, [68Ga]Ga-DPI-4452 imaging characteristics, dosimetry, pharmacokinetics, and safety were assessed in 3 patients with clear cell renal cell carcinoma. Methods: After [68Ga]Ga-DPI-4452 administration, patients underwent serial full-body PET/CT imaging. Blood and urine were sampled. Safety was monitored for 7 d after injection. Results: Tumor uptake was observed at all time points (15 min to 4 h). Across 36 lesions, the SUVmax at 1 h after administration ranged from 6.8 to 211.6 (mean, 64.6 [SD, 54.8]). The kidneys, liver, and bone marrow demonstrated low activity. [68Ga]Ga-DPI-4452 was rapidly eliminated from blood and urine. No clinically significant toxicity was observed. Conclusion: [68Ga]Ga-DPI-4452 showed exceptional tumor uptake in patients with clear cell renal cell carcinoma, with very high tumor-to-background ratios and no significant adverse events, suggesting potential diagnostic and patient selection applications.